INDEX
- Package contents
- Software installation
- Preparing the system
- Starting up
- Preparing the patient
- Performing the examination
- Power off and stand-by
- System management
- a. Safety information
- b. Copyright
Package contents

2. Teethan plate
3. Gel electrodes
4. USB receiver
5. USB extension cable
6. USB memory key with self-installing software and manual auto-installing
7. USB power cable
8. USB power supply
1. Software installation

Minimum requirements
Installation of the software requires a computer with a OS Windows 7 or higher with the following minimum requirements:
- Intel i5 Dual Core processor (or equivalent AMD), 4GB RAM
- 100 Mb free disk space
- 1 free USB 2.0 port
- Screen resolution 1280×768 or higher
- Internet connection
Installation
Insert the USB memory key included in the package to install the Teethan™ software. (see 1) Select the folder "Teethan installer" and double click on the file setup.exe; the installation wizard will start.
2. System preparation
Connecting the USB receiver
Connect the USB receiver to a USB port on your computer. (see 2)
Connecting the charging station
Charge the electromyography probes by connecting the charging station to a power source (PC or electrical source) using the USB cable and/or external power supply. (see 3) Each probe has an LED indicator (a blue light indicates that the probe is charging). When charging is complete the LED turns off.
Switching on the probes
Remove the probes from the charging station by unhooking being careful not to pull the cable connecting them. Activate the probes by gently swiping them on the "Swipe for activation" area. (see 4)
Rapid flashing of the LED indicates that the probe is searching for the USB receiver to connect to. A slower flashing indicates that the probe is connected.
3. Start
Open the program
The first time you run the program, you will be asked to enter the activation code issued upon confirmation of the contract. You will also need to be connected to Internet to enable automatic activation of the user licence and periodic updates.
System check
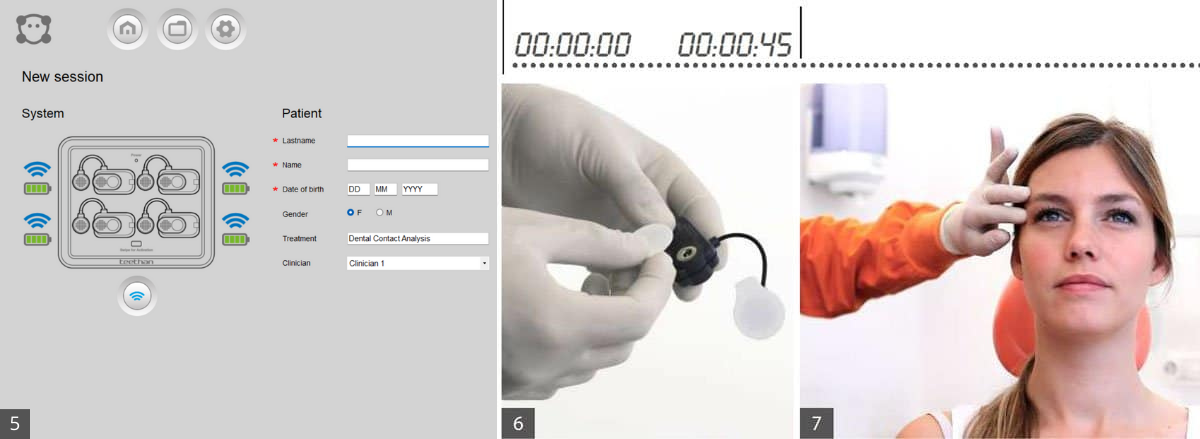
The initial screen of the application allows you to check the status of the system. (see 5) The battery charge level and the status of the probe/receiver connection are displayed next to each probe. The blue icon indicates that the probe is connected. If one or more probes are not connected (grey icon), press the search button to make a new connection. If the problem persists, it may be necessary to change the transmission channel frequency (refer to the manual in the 'Troubleshooting' section).
Start
When all probes are connected you can start the examination. Enter the patient data in the appropriate fields and click OK and the calibration screen will appear.
4. Patient preparation
Electrode attachment
Apply the gel electrodes to the probes onto the metallic clips and remove the protective film. (see 6)
Probe positioning
Place the probes on the patient's face onto masseter and temporalis muscles. Each probe is dedicated to a specific muscle, as indicated on the label:

Application of probes to the temporal muscles
To locate the anterior bundle of the temporalis, palpate the muscle by asking the patient to perform a maximum clenching. Locate the major axis of the zygomatic process of the frontal bone and apply the probe along the anterior margin of the muscle (close to the coronal suture 2cm from the zygomatic process). (see 7)
Application of probes to the masseter muscles
To locate the masseter, palpate the muscle in serratus and locate its belly. Apply the probe in a direction parallel to the course of the muscle fibres and in the central portion of the muscle (along the line joining the outer side of the eye with the mandibular angle). (see 8)
Symmetry and posture
It is advisable to maintain symmetry of positioning between the left and right sides. (see 9) To minimise any interference due to patient posture, make sure that the backrest of the chair is upright and place the patient in a relaxed position with legs uncrossed, hands resting on knees and eyes forward.

5. Performing the examination
Calibration test
Place two cotton rolls between the arches on the 5th and 6th teeth (second premolars and molars). (see 10) Ask the patient to clench the teeth as tightly as possible (see 11) and click on the "Rec" button to start the calibration, after 5 seconds the recording stops automatically. (see 12) You can perform and compare several calibration tests before moving on to the acquisition phase.
Acquisition
Remove cotton rolls. Ask the patient again to clench as hard as possible (allowing natural tooth intercuspation) and start the acquisition. (see 13) It is possible to take several consecutive acquisitions and then save only the selected ones.
Storage
Save acquisitions. (see 14) Files are automatically recorded in the archive with the name of the patient, the date of execution and the progressive acquisition number. Refer to the manual for data interpretation.

6. Power off and stand-by
Probe deactivation and recharging
At the end of the acquisition, switch off the probes by placing them for a moment on the activation surface (the white LED stops flashing) (see 15) or by attaching them directly on the charging station. (see 16) When the charging is complete, the system goes into stand-by and can remain permanently connected to the power supply.
Battery life
Teethan™ probes use Li-Poly batteries for their operation. The batteries provide approximately 7 hours of continuous acquisition time. For best performance, it is recommended to store the probes in the charging station every time you finish an acquisition. The batteries are equipped with a protection circuit for overvoltage, undervoltage and short circuits. They can only be replaced by Teethan™ personnel.
7. System management
Updates and Licence
Use of Teethan™ is governed by the user licence you have signed. In order to keep the system up-to-date and running, it is necessary to connect the computer to the internet at least once a month. The software automatically connects to the Teethan™ server and checks the status of the licence and the availability of any updates. If by the tenth day of each month Teethan™ is unable to establish a connection to the update centre, a warning will appear to remind you to activate the internet connection.
Cleaning
To clean the charging station, probes and receiver, use a dry cloth or one moistened with a neutral detergent. The devices must be kept dry. To avoid damage to the materials used, do not use alcohol, degreasers or chemical solvents. Since the parts do not come into direct contact with the patient (due to the interposition of the pre-cells), no sterilisation processes are necessary.
a. Safety information
It is recommended that any operation is carried out in accordance with the safety instructions in the manual on the USB memory key and at www.teethan.com. The safety of the instrument cannot be guaranteed if these conditions are not met. Teethan™ is a medical device (EU Directive 93/42/EC and its amendments, including Directive 2007/47/EC) which must always be used under the supervision of competent and authorised personnel, according to the regulations in force in the country where it is used. EMG probes are classified as ETSI EN 300 440 "Receiver category 3" according to the R&TTE Directive 99/5/EEC. Teethan™ must always be used only for this purpose, by qualified personnel, in environments suitable for performing electromyographic analyses, and in compliance with the regulations in force in the countries where it is used. Teethan™ is a device owned by Teethan SpA granted for use according to the conditions indicated in the rental agreement. The system software is supplied under a licence agreement and may not be copied, distributed or transferred to third parties. In case of failure, malfunction or replacement of the internal batteries please contact Teethan™ Customer Service. Please refer to the manual for terms of use, features and operating conditions.

The system contains batteries, in case of authorised disposal please observe the valid legal regulations. In accordance with Directive 2002/96/EC (WEEE) all equipment supplied after 13/08/2005 may not be disposed of with household waste. Teethan™ belongs to category 8 (Medical Devices) and is classified in the Business-to-Business sector. Disposal regulations may differ in individual EU countries.
b. Copyright
Teethan™ is a registered trademark of Teethan S.p.A., all other trademarks mentioned are registered trademarks of their respective owners. No part of this document may be copied or transmitted in any form or by any means, electronic or mechanical, including photocopying, without written permission from Teethan S.p.A. Unless otherwise specified, all references to companies, names, data and addresses used in the screen shots and examples are purely coincidental and are intended solely to illustrate the use of the product. This publication contains confidential information belonging to Teethan S.p.A. The technical characteristics and operating methods described may be subject to change without notice. Copyright © 2021 Teethan S.p.A. All rights reserved.
